Abstract
Investing in the mass production of Lateral Flow Assay (LFA) kits to identify active COVID-19 cases is a rational strategy to reopen the economy while keeping mortality low. With mass production, the majority of the workforce—or even entire populations—could be tested on a weekly basis.
Introduction
Despite spending trillions of dollars on military equipment in the name of national security, world leaders failed to adequately prepare for a global pandemic. The warning signs were there: SARS, H1N1, and other outbreaks over the last two decades highlighted the threat, yet little action was taken to minimize future loss of life.
In the absence of vaccines and effective treatments, governments have largely relied on two strategies:
a) Herd immunity (community immunity): Allowing the virus to spread naturally until a large portion of the population develops immunity. This approach keeps economies open but leads to high mortality.
b) Flattening the curve: Imposing lockdowns and restrictions to slow infection. This reduces mortality but forces economies into prolonged shutdowns.
Most countries have chosen the latter, hoping that vaccines or treatments would arrive quickly.
Yet after months of strict distancing, it has become clear that infections cannot be eliminated without new tools. In Canada, for example, 1,000–1,500 new cases were still being reported daily despite restrictions. While government support programs cushioned vulnerable families, such aid cannot last indefinitely. Optimistic forecasts placed vaccines or treatments at least 12 months away, while financial support was expected to last only a few months. This imbalance highlights the urgency of finding alternative strategies.
Discussion
The central challenge is to reduce active cases and deaths while avoiding economic collapse. Current preventive measures—distancing, masks, disinfection—are necessary because we do not know who is infected. But why should “unknown carriers” remain unknown when testing could identify them?
Imagine a community where every person carries proof of a recent negative test. In such a case, restrictive protective measures would no longer be needed for interactions between negative individuals.
There are two main testing methods for COVID-19:
RT-PCR: Detects viral RNA directly. It is accurate and can quantify viral load, but requires laboratories and takes hours to complete.
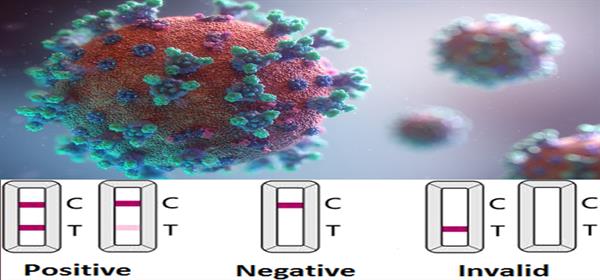
LFA (Lateral Flow Assay): Detects antibodies (IgG and IgM) from a drop of blood. Results are available within minutes, require no laboratory, and can be administered without professional supervision. It is inexpensive and can be produced at scale, comparable in simplicity to a pregnancy test or blood sugar test.
Because of its speed, affordability, and scalability, LFA is the most practical option for mass, repeated screening.
To make this system effective:
LFAs should be mass-produced at low cost (a few dollars each or less).
Tests should be available for free—or at minimal cost—at easily accessible locations such as gas stations, metro stations, and convenience stores.
Negative results should generate a temporary pass or ID, valid for a few days, required to enter workplaces and public venues.
RT-PCR tests could complement LFAs by tracking confirmed carriers until they test virus-free.
This system would reduce uncertainty, allow economies to reopen, and prevent mass shutdowns until vaccines or treatments are ready.
Conclusion
The mass production and deployment of inexpensive LFA kits offers a clear exit strategy for the COVID-19 pandemic. By identifying positive cases quickly and cheaply, societies can reduce mortality, eliminate uncertainty, and keep economies open without the need for prolonged national lockdowns.
Reza Parsa Nejad, PhD
April 21, 2020